Introduction: 25-30% of adult patients with AML have mutations in the isocitrate dehydrogenase (IDH) genes, IDH1 or IDH2. Hypomethylating agents (HMA) and venetoclax (Ven) is a widely used combination in induction-ineligible patients with IDH mutations. A subset analysis of VIALE-A data showed composite complete remission or complete remission with incomplete hematologic recovery (CR/CRi) rate of 79% and median overall survival (mOS) of 24.5 months with frontline azacitidine and Ven. IDH1-mutant and IDH2-mutant patients had CR/CRi rates of 66.7% and 86.0%, respectively. IDH1-mutant patients had mOS of 15.2 months and IDH2-mutant patients did not reach mOS. We report a single-center retrospective analysis of HMA/Ven in patients with IDH mutations.
Methods: This single-center retrospective study examined all patients with IDH mutations who received HMA/Ven from January 1, 2018 to June 30, 2023 at Memorial Sloan Kettering Cancer Center and summarizes their characteristics and outcomes. Demographics, adverse events, treatment course, and laboratory and transfusion data were retrospectively reviewed with institutional IRB approval.
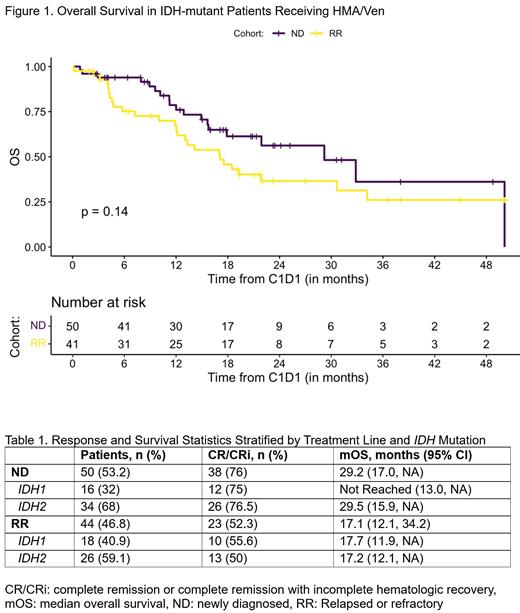
Results: Ninety-four patients, 50 with newly diagnosed (ND) AML and 44 with relapsed/refractory (R/R) AML, were identified (Table 1). 32% (n=16) of ND patients and 41% (n=18) of R/R patients had IDH1 mutations and 68% (n=34) of ND patients and 59% (n=26) of R/R patients had IDH2 mutations. With median follow-up of 21.9 months, mOS was 29.2 months (95% CI 17.9, NA) for ND patients and 17.1 months (95% CI 12.1, 34.2) for R/R patients (Figure 1). The CR/CRi rate was 76% (n=38; 30% CR, n=15; 46% CRi, n=23) for ND patients and 52% (n=23; 32% CR, n=14; 20% CRi, n=9) for R/R patients.
Patients received median of 3 cycles of HMA/Ven (range 1, 22) and experienced frequent treatment modifications and delays. Of 356 total cycles, 62% (n=218) were modified to have fewer than 28 days of venetoclax, 7 days of azacitidine, or 5 days of decitabine. In addition, 296 cycle holds or delays of at least one week occurred. 66.2% (n=196) of holds or delays were due to cytopenia and 11.5% (n=34) were due to infection. 77% (n=72) of patients had Common Terminology Criteria for Adverse Events (CTCAE) grade 3 pancytopenia and 47% (n=44) had febrile neutropenia. Through 356 cycles spanning 21,386 patient-days, 5,651 complete blood counts (CBCs, mean 15.9 per cycle), 986 red blood cell transfusions, (RBC, mean 2.8 per cycle) and 1,018 platelet transfusions (PLT, mean 2.9 per cycle) were recorded at our center. Additional CBCs or transfusions performed externally were not captured.
After CR/CRi, dose adjustments, cycle holds or delays, and cytopenias remained frequent. The 63 patients that achieved CR/CRi underwent 198 cycles, 71% (n=141) of which were modified as above. There were 209 holds or delays (65%, n=136 for cytopenias, 10%, n=21 for infection). Median time between cycles was 7 weeks. Cycles given after CR/CRi frequently resulted in grade 3 cytopenias by CTCAE (neutropenia: 82%, n=163; anemia: 15%, n=29; thrombocytopenia: 37%, n=74). Through 198 cycles spanning 13,463 patient-days, 2,426 CBCs (mean 12.3 per cycle), 141 RBC transfusions, (mean 0.7 per cycle) and 236 PLT transfusions (mean 1.2 per cycle) were performed.
At last follow-up, 28% (n=14) of ND AML patients continued HMA/Ven, whereas 28% (n=14) had proceeded to allogeneic stem cell transplantation (alloSCT), 28% (n=14) were relapsed or refractory to HMA/Ven, and 12% (n=6) were deceased. For R/R AML patients, 20% (n=9) continued HMA/Ven but 27% (n=12) had proceeded to alloSCT, 34% (n=15) were relapsed or refractory, and 14% (n=6) were deceased. Of the 68 patients who were no longer receiving HMA/Ven at end of follow-up, only 23.5% (n=16) of patients received an IDH-inhibitor in their next line of therapy, the rest primarily transitioning to alloSCT (38.2%, n=26), chemotherapy (8.8%, n=6), or supportive measures (20.6%, n=14).
Conclusion: In a single-center retrospective setting, HMA/Ven is efficacious in achieving CR/CRi in ND and R/R IDH-mutant AML. However, its use, even in CR/CRi, is associated with significant cytopenias and infections that necessitate indefinite and frequent treatment modification, laboratory utilization, and transfusions. A strategy that incorporates HMA/Ven followed by non-myelosuppressive targeted therapy with IDH inhibition as maintenance should be explored to minimize these toxicities.
Disclosures
Geyer:Novartis: Consultancy; Sanofi: Consultancy, Research Funding; Actinium Pharmaceuticals, Inc: Research Funding; Amgen: Research Funding. Goldberg:Genentech: Consultancy; DAVA Oncology: Honoraria; Trillium: Research Funding; AROG: Research Funding; Pfizer: Research Funding; Aptose: Research Funding; Aprea: Research Funding; ADC Therapeutics: Research Funding; Celularity: Research Funding; Prelude: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Astellas Pharma: Consultancy; Abbvie: Consultancy, Research Funding. Rampal:Ryvu: Research Funding; Constellation: Research Funding; Stemline: Research Funding; Zentalis: Research Funding; Incyte: Research Funding; Karyopharm: Consultancy; Zentalis: Consultancy; Servier: Consultancy; Kartos: Consultancy; Dainippon: Consultancy; Sumitomo: Consultancy; Morphosys/Constellation: Consultancy; Pharmaessentia: Consultancy; Galecto: Consultancy; CTI BioPharma Corp: Consultancy; Celgene-BMS: Consultancy; GSK-Sierra: Consultancy; Incyte: Consultancy. Park:BeiGene: Consultancy; Genentech, Inc.: Research Funding; Autolus Therapeutics: Research Funding; Amgen: Consultancy; Be Biopharma: Consultancy; Fate Therapeutics: Research Funding; Allogene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Research Funding; Intella: Consultancy; Takeda: Consultancy, Research Funding; Sobi: Consultancy, Research Funding; Pfizer: Consultancy; Minerva Bio: Consultancy; Affyimmune: Consultancy; Artiva Biotherapeutics: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Bright Pharmacetuicals: Consultancy; Curocell: Consultancy; Kite: Consultancy; GC Cell: Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding. Roeker:DAVA: Other: CME speaker; PeerView: Other: CME speaker; Curio: Other: CME speaker; Medscape: Other: CME speaker; AbbVie: Consultancy, Research Funding; Ascentage: Consultancy; AstraZeneca: Consultancy, Research Funding; Beigene: Consultancy; Janssen: Consultancy; Loxo Oncology: Consultancy, Other: travel support, Research Funding; Pharmacyclics: Consultancy; Pfizer: Consultancy, Research Funding; TG Therapeutics: Consultancy; Abbott Laboratories: Current equity holder in publicly-traded company; Adaptive Biotechnologies: Research Funding; Genentech: Research Funding; Aptose Biosciences: Research Funding; Dren Bio: Research Funding; Qilu Puget Sound Biotherapeutics: Research Funding. Tallman:Innate Pharmaceuticals: Consultancy; Foghorn: Membership on an entity's Board of Directors or advisory committees; Orsenix: Research Funding; UpToDate: Patents & Royalties; AbbVie: Research Funding; BioSight: Research Funding; Glycomimetics: Research Funding; Amgen: Research Funding; Rafael Pharmaceuticals: Research Funding; Novartis: Consultancy; Kura: Consultancy; Ipsen Biopharmaceuticalas: Consultancy; HOVON: Membership on an entity's Board of Directors or advisory committees; American Society of Hematology: Honoraria, Membership on an entity's Board of Directors or advisory committees. Stein:Bristol Myers Squib: Consultancy, Research Funding; Gilead: Consultancy; Calithera: Consultancy; Syros: Consultancy; Daiichi: Consultancy; Menarini: Consultancy; OnCusp: Consultancy; Syndax: Consultancy; Aptose: Consultancy; Servier: Consultancy; Foghorn: Consultancy; CTI Biopharma: Consultancy; Neoleukin: Consultancy; Astellas: Consultancy; Ono Pharma: Consultancy; Blueprint: Consultancy; PinotBio: Consultancy; Novartis: Consultancy; Janssen: Consultancy; Agios: Consultancy; Jazz: Consultancy; Genentech: Consultancy; Genesis: Consultancy; Abbvie: Consultancy; Eisai: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal